
|
Медико-биологический
информационный портал
|
|
для специалистов |
|
ТОМ 4, СТ. 129 (стр. 451-460) //
ETHANOL AND TOXICITY OF 1,2-DICHLOROETHANE Yuri Yu. Bonitenko, Evgeni Yu. Bonitenko All-Russian Center of Emergency and Radiation Medicine EMERCOM of Russia,
Abstract 128 patients severely poisoned by 1,2-dichloroethane (DCE) were divided into 3 subgroups: 1) patients poisoned with DCE and alcohol (Group I; n=42); 2) patients with history of chronic alcoholism poisoned with DCE but with no signs of acute alcoholism (Group II; n=38) and 3) patients poisoned with DCE only (Group III; n=48). The least favorable course of poisonings in respect to the lethal outcome was observed in Group II. In the experiment on adult male rats the influence of different modes of ethanol administration on DCE toxicity was studied. The long-term pretreatment of animals by ethanol resulted in the lowering of LD50 of DCE and decreasing of its concentration in blood. The administration of ethanol during a day after the poisoning leads to the increase of LD50 and concentration of both DCE and 2-chloroethanol in blood. The obtained data were analyzed regarding the influence of ethanol on DCE-biotransformation.
Keywords: poisonings acute, 1,2-dichloroethane, 2-chloroethanol, ethyl alcohol Introduction The acute poisonings with 1,2-dichloroethane (DCE) is still the urgent problem of the modern clinical toxicology, despite the fact that in the last years the number of cases of intoxication had considerably decreased. The reasons for the permanent concern about this problem are its considerable gravity and high lethality of the most frequent peroral poisonings with DCE. The minimum lethal dose of a poison for the adult person ranges from 10 to 30 ml [1,2], and is approximately equal to one swallow. It is the probable reason why the intoxications with DCE are predominantly grave. According to our data, which agree with some literature data, the percentage of mild, intermediate and heavy degree poisonings are 16, 10 and 74% respectively. The results of the treatment could hardly be classified as satisfactory. According to data by Luzhnikov [2], the hospital mortality in cases of poisonings by DCE compounds is about 50%. This figure is even higher in cases of heavy intoxication - 80% and higher, as a rule. The poisonings with DCE quite often developed in the presence of alcohol intoxication. According to our data, this is observed approximately in 30 - 40% of cases. The generally accepted view is that ethanol considerably increases the toxicity of the chlorinated hydrocarbons, including DCE [3,4]. The clinical experience not always confirms this point. Besides, it is now considered [2,5], that the toxicity of DCE is at least partially determined by its biotransformation to 2-chloroethanol (CE), and the toxicity of the latter and its metabolism in the body is determined by the same enzyme systems that determine the biotransformation of ethanol. Taking this into account, more detailed research of the influence of ethanol on toxicity of DCE is amply justified. Methods The study material obtained was based on clinical and experimental research. 128 cases of acute heavy degree peroral poisonings with DCE have been analyzed. All subjects were men aged 18 - 60 years, the average being 38,2 +/- 5,6 years. All the patients with intoxication by DCE combined with other exogenous poisons (except ethanol), mechanical and thermal traumas, cases of intoxications by DCE in patients with diabetes, drug abuse, III stage chronic alcoholism, and long-term administration of some medications (psychopharmacological drugs, cimetidinum etc.) were eliminated from the study. The diagnosis of poisoning was done based on the history findings, clinical course, and the laboratory confirmation of the remains of poison. The medical procedures included manual resuscitation as recommended, elimination of poison from the gastrointestinal tract, early haemodialysis or haemoperfusion, forced diuresis, infusion therapy with the use of pathogenetic and symptomatic means. All the patients were divided into three subgroups: those poisoned with DCE in the presence of alcoholic (concentration of ethanol in blood more than 0,5 g/l), - Group I - 42; patients suffering from I - II stage alcoholism, but whose DCE poisoning was not associated with acute alcoholism or intoxication, (concentration of ethanol in a blood less than 0,5 g/l) - Group II - 38. In the Group III there were 48 patients, in whom the poisoning with DCE was not combined with alcoholic intoxication and/or with chronic alcoholism. The above groups did not differ significantly by the age, duration and extent of medical care. The experimental part of the research was done on white male rats (mass 180 - 220 g.). DCE was administered in an oil solution in a single dose into the stomach. The animal were divided into several groups: the control ones received only DCE; the second group of animals was given 20% ethanol solution for a month instead of drinking water (12 hours prior to DCE administration, ethanol solution was replaced with water); in the third group 30 min before the DCE was given ethanol was administered in a single dose (3 ml/kg in 30% solution); in the animals of the fourth and fifth groups ethanol (in 30% solution) was injected 1 hour after DCE was given intraperitoneally in the doses of 0,5 ml/kg, and 2 ml/kg respectively (the first three injections, further in dose 1,5 ml/kg) every 4 hours during the first day following DCE administration; the ethanol concentration in animal blood (for doses 0,5 ml/kg,) ranged daily from 0,75 up to 0,3 g/l, while in the second group - animals given single dose of alcohol 2 ml/kg - the ethanol concentration varied from 3,2 to 1,8 g/l. The survival rate and the study results as well as the concentration of poison and its metabolite CE in blood of the animals was analyzed. The concentrations of DCE, CE and ethanol were measured by gas chromatography, using "Zvet-500" series. The identification of ethanol was carried out by traditional method. For identification and quantitative analysis of DCE the ionization-flame detector (IFD) was used; to determine CE, as well as IFD, electron capture detector was also employed. Helium was used as the gas-carrier. Feed rate of hydrogen and helium was 30 ml/min, and for air - 300 ml/min. As a filling of the columns in the DCE analysis Chromaton N-AW, 0,250 - 0,315 mm was used, saturated with 15% Scvalan, Chromaton N-AW-DNCS, 0,160 - 0,200 mm, saturated with 15% Reaplex - 400, Chromaton N-AW, 0,200 - 0,250 mms, saturated with Carbovacx - 6000. The temperature of pillars was 70 - 90oC, evaporator - 130oC. To isolate CE, Chromaton N-AW, 0,200 - 0,250 mms, saturated by 15% polyethylene glycol, was applied. The temperature of the pillar was 150oC, and of the evaporator - 170oC. The mortality rate (LD50) has been calculated with the help of probit analysis [6], the clinical study results have been assessed by c2, and the experimental results have been quantitatively evaluated according to Student—s T-test. Results When analyzing clinical examination findings (Tab.1) Table 1 The clinical characteristic of poisonings with 1,2-dichloroethane
As for clinical manifestations, encephalopathy in a toxicogenic phase is registered in all cases of heavy poisonings. When poisoning with DCE is accompanied by alcoholic intoxication the rate of severe disorders in consciousness is the highest, while in more than a half of the patients of Group II (55,2%) the cerebral disorders of the initial period are limited by short-term disorders - that is manifestations similar to those of average degree DCE poisoning of, and a clinical picture of severe intoxication becomes further evident. Marked manifestations of encephalopathy in patients of Group I are demonstrated by acute respiratory insufficiency (ARI). ARI of central and aspiration-occlusive genesis (40,4%) predominates in patients of Group II while for those suffering from chronic alcoholism similar disorders have been less often (13,1%) observed. The proportion of individuals suffering from primary toxicogenic collapse is the highest in patients of Group I. This complication is primarily found in cases, when the ethanol concentration in blood is about 2,5 - 3,0 g/l or more and indicates the ability of ethanol to enhance cardiovascular disorders in case of poisoning with chlorinated hydrocarbons. As for exotoxic shock, most frequently it has been registered in patients suffering from chronic alcoholism. In the same group the proportion of persons who develop marked consumption coagulopathy is the highest. Gastrointestinal disturbances have been observed in all the patients, and the incidence of some forms of these disturbances practically does not differ in various groups. Since most of those severly poisoned with DCE die on the 1st - 2nd day of intoxication, there is not enough time for the evident clinical manifestations of hepatopathy to develop, and morphological studies practically in all patients who died within 8 to 12 hours after poison intake or later, show similar pattern of hepatocytes dystrophy and centrylobular necrosis. To assess the degree of hepatopathy, we used the rates of aminotranspherases (AsAT, AlAT) serum activity. The increase of these rates 2 and more times in 6 - 12 hours after poison intake, the dominant AsAT activity, enzyme activity increase 5 and more times by the end of the 1st day, were considered to be of importance. Enzymologic shifts have been found in all the control groups which occurred with approximately the same frequency. These shifts have been estimated as significant in 76% of cases in Group II, whereas in other control groups this finding was at the level of 48 - 50%. The hospital mortality rate seemed to be the highest in Group II. Under the severe poisoning with DCE three peaks of mortality rate have been observed: during the first 4 - 6 hours following the poison intake, in the second half of the 1st and 2nd day, and at the end of the first week of intoxication. Rapid death is mainly associated with deep coma, respiratory failure, toxicogenic collapse and is more common in combination of DCE poisoning and alcoholic intoxication. The unfavorable outcome at the end of the first - the beginning of the second day is primarily due to exotoxic shock, and is more common for patients, suffering from alcoholism, and rarely - in the "true" DCE poisoning and most rarely in case of combination of DCE and ethanol. Death at the end of the first - beginning of the second week was, mainly, due to acute hepatic failure, and was rather uncommon (in 10% of the total number of lethal outcomes), and mortality rate did not essentially differ in various groups. On admission of DCE concentrations in blood of the poisoned patients, ranged from 0,3 to 5,0 mmol/l, the average values being approximately the same in different groups of patients. In the first 2 - 3 hours following the DCE intake biological media of those poisoned showed either no CE or its traces only. The data dynamics of a chemical toxicological blood test in later periods was not characteristic, mainly as a result of intensive eliminative therapy. The chemical toxicological findings when compared with early clinical manifestations in DCE intoxication suggest that narcotic poison concentration is at the level of 0,6 - 0,8 mmol/l; it reaches 1,0 mmol/l in alcohol addicts, while reducing down to 0,4 - 0,5 mmol/l in case when alcoholic intoxication is combined with poisoning. Thus, the analysis of clinical data allows to suppose, that DCE poisoning combined with alcoholism are the least favorable; that is at the lowest doses of poison the patients of this category develop exotoxic shock more often, than those in the other groups; besides hepatocellular lesions develop more rapidly and the hospital mortality is the highest. The patients suffering from DCE poisoning accompanied by alcoholic intoxication have quite different characteristics. The pronounced initial encephalopathy occurs most often, which is associated with central, aspiration obstructive and mixed respiratory disorders and primary toxicogenic shock. At the same time exotoxic shock develops less often and, as has been pointed out, the mortality rate is not higher than that in a group of patients whose poisoning was not combined with alcohol intake it seems likely that the view on enhancement of DCE toxicity in the presence of ethanol is mainly associated with more pronounced initial cerebral disorders, respiratory and cardiovascular disturbances caused by nonelectrolyte effects of 1,2-dichlorethane. To estimate the influence of ethanol on DCE toxicity under standard conditions the experimental study was carried out. The results of the experiments on the animals poisoned with DCE (Tab.2), Table 2 Influence of different modes of ethanol administration on toxicity of DCE in rats.
allow to conclude, that in groups simulating a clinical situation (I - control, II - receiving ethanol for a long period before poisoning, III - receiving ethanol in a single dose before injection of DCE) the changes similar to those for patients of similar groups are observed. Thus, the values of LD50 are the lowest in Group II, and for animals of Groups I and III they do not essentially differ. The duration of a lateral position (after administration of DCE dose 1,5LD50 of a control) is the least for rats, pretreated by long-term administration of ethanol, and the greatest (in Groups I - III) for animals, receiving ethanol in a single dose before administering DCE. In the second group the most rapid lethal outcomes have been noted. The results of experiments, in which ethanol was administered after poisoning with DCE in small (Group IV) and large (Group V.) doses present some interest. The findings of Group IV did not considerably differ from the control ones. For animals of Group V the most apparent differences from the control data were detected: the highest values of LD50, the longest lateral position and late development of lethal outcome (following administration of DCE in a dose 1,5LD50 of the control). The determination of DCE concentration in blood also shows the most pronounced differences in animals of Groups II and V (Tab.3), and the data of Group II are lower, and of Group V - higher, than those in the control group. Besides, in Group III the tendency to higher level of DCE, especially in 3 hours after poisoning has been noted, but differences from the control data are supposed not to be true. The values of chloroethanol (Tab.4) in Group V are much higher than those in the control group, in Groups III and IV these appear to be only in 3 and 12 hours respectively following poisoning; CE concentration in the blood of animals of Group II in all points of determination is somewhat lower than that in the control group, but the difference is not statistically reliable. Table 3 Concentration of DCE (mM/l) in blood of poisoned rats.
* - p < 0,05: ** - p < 0,01 - 0.001 vs Group I Table 4 Concentration of CE (mн/l) in blood of poisoned rats.
* - Т < 0,05: ** - Т < 0,01 -0.001 vs Group I Thus, summarizing up the results of the experiments, the data obtained can be generalized as follows: animals, receiving ethanol in a single dose before DCE intake or in small doses within a day after it do not demonstrate findings different from those of the control group. The long-term pretreatment of animals with ethanol leads to a considerable increase in toxic action of DCE and more rapid decrease in concentration of the unchanged poison in blood. The administration of high doses of ethanol following DCE is accompanied by the decrease in lethal action of the latter in the presence of marked increase of narcotic effects, increase in concentration of unchanged dichloroethane in blood and, especially, its metabolite - chloroethanol. Discussion Comparing the clinical data, obtained, with materials by other authors, who studied this problem, we can note their coincidence in a number of details, however, with some difference in general estimation. So, according to Belyakov [3], the combination of DCE poisoning with the use of ethanol results in the increase in the rate of lethal outcomes in the first 4 hours in case of agony and coma, while in "true" DCE poisoning the main cause of deaths is acute cardiovascular insufficiency. According to our data the combination of DCE with ethanol causes increase in the depth of coma, cerebral and aspiration-obstructive disorders of respiration and is followed by the tendency to increase the frequency of toxicogenic collapse in the early period of intoxication, but less often results in exotoxic shock. The author cited above concludes that there is a considerable increase in DCE toxicity in combination with ethanol. It is our opinion that ethanol increases those early manifestations of DCE intoxication, which are due to narcotic effects of the poison; however it does not cause marked changes in the rate of its toxicity - hospital mortality rate. It is advisable to interpret the results of clinical and experimental research based on the existing understanding of the mechanisms of DCE action. At present, the DCE toxicity is considered to be due to both intact molecule of poison and products of its metabolism in a body. The action of DCE is mainly responsible for early cerebral disorders caused by narcotic effects of poison, as well as cardiodepression, hemolysis etc. However, the main role in toxic action DCE is assigned to its metabolites (Fig.1). 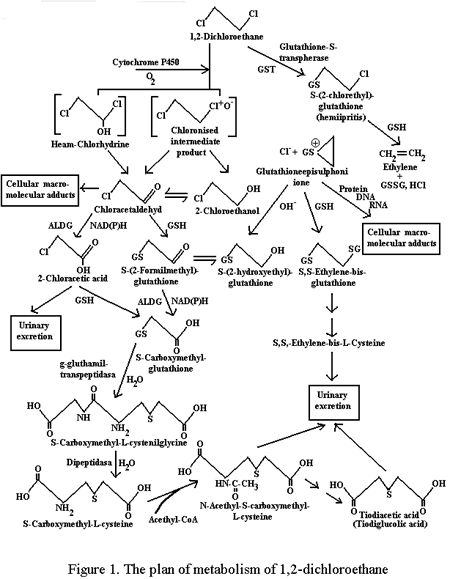
Initial phase of DCE metabolism is its lysis with the participation of two enzymatic systems: nonspecific monooxygenase system of microcoms (NMSM), associated with cytochrome P450 and cytosol glutathion-S-transpherase (G-S-T), the main role being assigned to NMSM. The interaction of DCE with G-S-T results in the formation of hemiipritis and glutathionepisulphonium ion possessing alkylation properties. This metabolic process determines primarily the damage to DNA and does not obviously play the important role in the acute toxicity of poison. Under the influence of NMSM, the formation of heamchlorhydrinum and chloronised intermediate product (reactive galozocomplex) is possible. These compounds through the way other than enzymatic, are transformed into 2-chloroacetaldehyde (CAA) and 2-chloroethanol, which, in turn, under the influence of alcohol (ADG)) and aldehyddehydrogenase (AlDG) transform into monochloroacetic acid (MCAA). CAA and MCAA when combined with recovered glutathione dechlorinate, transforming into products of low biological activity. At one of the stages of these transformations, the dechlorised derivative of CAA, combined with glutathion, is metabolized by AlDG. The above scheme of DCE metabolism in a body is presently the most recognized. At the same time the opinions of different authors on which of the formed DCE biotransformation products determine its toxicity, do not always coincide. So, according to Mizyukova and Kokarovtzeva [7], CAA and MCAA are considered to be similar metabolites, while Novikovskaya and Lesovik [8] believe activation of peroxide oxidizing lipids (POL) to be the main process which can be initiated by intermediate products of DCE metabolism, for example by reactive galozocomplex. Considering the above mentioned processes from the point of view of the possible effect of different modes of ethanol entering a body, several links in a metabolic chain can be distinguished, where this effect is the most probable, having at the same time multiple targets under long-term previous use of ethanol and its presence in the bioenvironments on the background of DCE intoxication. It is the first stage of NMSM metabolism that we are speaking about. Long-term pretreatment by ethanol causes induction of NMSM, which is proved by the decrease of hexenalum sleep duration (in our experiments - with 32,8 +/- 2,6 in the control group up to 20,6 +/- 1,8 min; p < 0,01). The influence of this induction on DCE metabolism is confirmed by the low poison concentrations in blood of the animals in the given group, as well as by the increase of their mortality. One can not completely exclude the role of induction of microsomal ethanol oxidizing system (MEOS), which is known [9] to be observed with long-term administration of ethanol. This can be important for the acceleration of CE biotransformation. Above phenomenon, however, requires further study since the involvement of MEOS in CE biotransformation could not be accurately identified. It is quite different when DCE and ethanol are simultaneously present in the bioenvironment. Under similar conditions ethanol is capable to enhance the non-electrolyte effects of DCE. Another effect of ethanol on DCE metabolism seems to be possible - by means of inhibition of CE transformation into CAA and further into MCAA. Ethanol ability to compete with CE for ADG does not cause any doubts and is confirmed by literature data [10, 11, 12]. Ethanol ability to inhibit CE metabolism is also confirmed by the present study results - for animals of Group V poisoned with DCE, CE concentration in blood in the presence of administration of high doses of ethanol was much higher, than in the control group. It seems to be more difficult to evaluate the effect of acetaldehyde generated during ethanol metabolism on DCE biotransformation. Most probably, this influence can be exerted at the stage of transformation of CAA into MCAA by way of competition of two aldehydes for AlDG. The data, presented in the given paper, do not allow to evaluate this possibility. At the same time, its study is supposed to be useful. The results obtained prove, though indirectly, the influence of calcium Cyanamid (inhibitor of AlDG) in combination with amide of isovaleric acid (inhibitor of ADG) on DCE toxicity [13] - the use of similar combination in experiment results in the essential increase of the LD50 of dichloroethane finding. Certainly, it would have been great simplification to reduce the influence of different modes of ethanol administration on DCE toxicity to the effects, associated exclusively with the discussed above possibilities of modification of poison toxicokinetics and its metabolites and not to take into consideration numerous and diverse biochemical and functional changes caused by ethanol. The acute and chronic use of ethanol is known to stimulate POL, casing redistribution of oxidized and reduced forms of coenzymes, and exerting an action on the enzymes of glutathion metabolism, the contents of -SH and S-S forms etc. [14, 15, 16]. However, the data obtained and literature data [17,18,19], allow us to conclude that as far as the ethanol effect on DCE toxicity is concerned along with non-electrolyte effects, the important role is played by microsomal metabolism modification and the effect of ethanol on transformation of chloroethanol into monochloroacetic acid, the latter essentially determining the specificity of the poison action. References 1. Bandman A.L., Voytenko G.A., Volkova N.V. et al. Harmful chemical agents. Hydrocarbons. Galogene derivatives of hydrocarbons. St-Petersburg: Chemistry, 1990: 1-732 2. Luzhnikov е.б. Clinical toxicology. Moscow: Medicine, 1994: 1-256 3. Belyakov V.P. On the effect of ethanol on the course of dichloroethane poisonings. In book of abstracts: Second All-Russian Congress of Forensic Physicians. 1987: Irkutsk.: 224-5 4. Demina V.I., Merculova V.I. On forensic expertise of poisonings combined with alcohol. In book of abstracts: Second All-Russian Congress of Forensic Physicians. 1987: Irkutsk.: 219-20 5. 1,2 - Dichloroethane. Health and Safety guide 1990; 62: 1-80 6. Prozorovski V.B., Prozorovskaya M.P., Demuenko V.M. Rapid test of determination of an average effective dose and its errors. Pharmacol Toxicol 1978; 41(4): 497-502 7. Mizyukova I.G., Kokarovtzeva M.G. Medical efficiency of acetylcysteinum in acute poisoning with dichloroethane. Pharmacol Toxicol 1978; 41(3): 350-4 8. Novikovskaya T.V., Lesovik J.A. Specific therapy in acute dichloroethane poisoning. In book of abstracts: The proceedings of Moscow Research Institute of Emergency Medical Care. 1988: Moscow: 74; 106-12 9. Lakin K.M., Krylov F.U. Biotransformation of medications. Moscow: Medicine, 1981: 1-344 10. Bonitenko Y.Y., Bocharov N.V., Lishenko V.V. The effect of some findings of ethylenechlorhydrinum toxicity. Hygiene Transactions Prof Diseas 1981; 9: 44-5 11. Johnson M.K. Metabolism of chloroethanol in the rats. Biochem Pharmacol 1967; 16 (1): 185 12. Peterson D.I., Peterson J.E., Hardinge V.G. Protection by ethanol against the toxic effects of monofluoroethanol and monochloroethanol. J Pharmacy Pharmacol 1968; 20 (6): 465-8 13. Bonitenko Y.Y., Bonitenko E.Y., Poisoning with alcohols - some clinical and experimental aspects. Arch Toxicol Kinet Xenobiot Metab 1998; 6 (3): 341-6 14. Novikov N.G. Oxidizing processes disorders in heart and liver in ethanol intoxication and their correction by glutamate and succinate. Essay of PhD. dissertation. 1985: Chelyabinsk; 1-25 15. Sippel H.W. Effect of an acute dose of ethanol on lipid peroxidation and on the activity of microsomal glutathion-S-transferase. Acta Pharmacol Toxicol 1983; 53 (2): 135-40 16. Yuki T., Yoshitoku K., Nakagava Y., Ohashi K. et. al. Changes in hepatic lipid peroxidation after acute and chronic ethanol treatment. In book of abstracts: Med Biochem Chem Aspects Free Radicals. 1989: Amsterdam: 967-70 17. Guengerich F.P., Crawford W.V., Domaradzki J.Y., Macdonald T.L., Watanabe P.G. In vitro activation of 2-dichloroethane by microsomal and cytosolic enzymes. Toxicol Appl Pharmacol 1980; 55: 303-17 18. Shilov V.V., Plugnikov N.N., Sofronov G.A. Modification of toxicokinetics and toxicodynamics of dichloroethane with the use of perfluorine. In book of abstracts: Perfluoranhydrocarbon compounds in biology and medicine 1997; 8: 46-52 19. Shilov V.V. Detoxification therapy of acute poisonings by lipophilic xenobiotics with the help of perfluoranhydrocarbon compounds. Essay of MD. dissertation. 1999: S-Petersburg; 1-43 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Свидетельство о регистрации сетевого электронного научного издания N 077 от 29.11.2006 Свидетельство о регистрации сетевого электронного научного издания N 077 от 29.11.2006
Свидетельство о регистрации сетевого электронного научного издания Медлайн.Ру - N 227 от 20 октября 2008 года.
Выдано ФГУП "Информрегистр" Журнал основан 16 ноября 2000г.
Выдано Министерством РФ по делам печати, телерадиовещания и средств массовых коммуникаций
(c) Перепечатка материалов сайта Medline.Ru возможна только с письменного разрешения редакции
|
|
| Размещение рекламы | |
|
| |

 Articles
Articles
