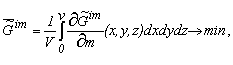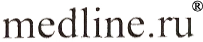|
|
СОДЕРЖАНИЕ ЖУРНАЛА:
Адрес редакции и реквизиты
192012, Санкт-Петербург, ул.Бабушкина, д.82 к.2, литера А, кв.378
Свидетельство о регистрации электронного периодического издания ЭЛ № ФС 77-37726 от 13.10.2009
Выдано - Роскомнадзор
ISSN 1999-6314
|
|
 |
|
ТОМ 3, СТ. 28 (стр. 260) // Ноябрь, 2002 г.
THERMODYNAMICS OF BIOLOGICAL EVOLUTION AND AGING
Г.П.Гладышев, 2001
УДК 536.7+531.3:541.1:575.8001.57; 576.1:577:577.3+577.1+577.7+577.42
Georgi P. Gladyshev
Supramolecular thermodynamics is a key to understanding phenomena of life. What is life from a physical chemist's viewpoint
"Успехи геронтологии", 2002г., выпуск 9
International Academy of Creative Endeavors, Moscow, Russia, San Diego, USA;
N. Semenov Institute of Chemical Physics, Russian Academy of Sciences,
Kosygina 4, Moscow, 117977 Russia; http://www.endeav.org/evolut
E-mail: academy@endeav.org
"One of the principal objects of theoretical research in any department of knowledge is to find the point of view from which the subject appears in its greatest simplicity."
J. Willard Gibbs (1881)
The law of temporal hierarchies of the biological world allows us to pick out of the biomass quasi-closed thermodynamic systems with a given hierarchy. It has been established, that the use of this law of Nature as applied to supramolecular structures of organisms allows us the opportunity of using the methods of equilibrium supramolecular thermodynamics in the examination of open living systems.
It has been shown that supramolecular thermodynamics is one of the "keys", which allows us to explain the origin of life and evolution of living beings.
The second law of thermodynamics in its classic formulation (R.Clausius & J.W.Gibbs) is easy to apply in order to make calculations, carried out through methods of chemical, supramolecular and overall hierarchical thermodynamics.
Key words: supramolecular thermodynamics, thermodynamics of aging, thermodynamics of biological evolution, law of temporal hierarchies.
Introduction
J. Willard Gibbs created the general thermodynamic theory, which is a strict physical theory applying to the whole real world. This theory has been of limited or questionable use in biology for the investigation of open systems.
Recently the Gibbs' theory has been extended to empirical open biological systems and a hierarchical thermodynamics has been created. A study of quasi-closed systems enables one to make conclusions about the thermodynamic direction of biological evolution and aging of living beings. The most essential application of the theory is the study of living creatures' behavior and anti-aging medicine, gerontology, pharmacology, nutrition and other branches of biology and medicine [1-10].
General
During the last decades an opinion has widely spread that there is "an apparent contradiction between biological order and laws of physics - particularly the second law of thermodynamics". Besides, it is claimed that this contradiction "cannot be removed as long as one tries to understand living systems by the methods of equilibrium thermodynamics".
The author of the present work states: if living systems are described in the framework of hierarchic equilibrium thermodynamics, this contradiction does not exist.
Recently the foundations of equilibrium hierarchical thermodynamics of heterogeneous biological systems have been created.
A model of the origin and evolution of life has been proposed. This model investigates the phenomena of life as processes of quasi-equilibrium thermodynamic self-organization (spontaneous self-assembly) of self-reproduced biological structures. The law of temporal hierarchies has been formulated [1-10].
A look at the world around us shows that the average lifetimes (life spans) of small bio-molecules, macromolecules, supramolecular structures, cells in tissues; organisms; and populations have a number of strong inequalities.
For example, the lifetimes of amino acids in tissues (from the moment they appear in the cell up to the time of their participation in chemical transformations) are substantially shorter than the lifetimes of protein macromolecules in cells. In turn, macromolecules exist in bio-tissue cells for a short time in comparison with the lifetimes of the cells themselves. The cells (in many cases) live for a significantly shorter period than the lifetime of the organism, and the lifetime of the organism is a lot shorter than the lifetime of the populations they create.
It is clear that the indicated conformity to natural laws lays down the conditions for the possibility of the metabolism.
This conformity to natural laws cannot be deduced from any well-known principles. From this, it follows, that the series
| |
tm << tim << torganell << tcel << torg << tpop << tsoc |
(1) |
is an independent general law of nature. Here t - average lifetime of "free" molecules-metabolites (m), supramolecular structures (im), organelles (organell), cells in the tissue (cel); organisms (org); populations (pop); societies (soc).
As a matter of fact, the series (1) is an expression of the regularity that hierarchic structures have essentially different lifetimes.
However, these structural types are not general for all bio-systems. For instance, it is possible that certain cells (nerve cells, heart muscle cells) are not renewed throughout the human life. These cells are, as if, not cells in the usual sense; in this case, tcel (for these cells) should be removed from the series (1). A similar phenomenon is observed for the fruit fly: no cell in the adult fly body undergoes division. Likewise, the proteins of the animal eye lens are almost never renewed. In this case, the lifetimes of these macromolecules do not fit the series (1) either. The space hierarchy does not match the temporal hierarchy in the above examples. In such a case, the corresponding lifetimes of the structures are as though involved in the next temporal hierarchy.
It is easily shown that the existence of series (1) allows us to pick out the summation of structures of one hierarchy as a subsystem and to consider this subsystem as a quasi-closed system. In order to study such a system, it is possible to use the methods of equilibrium hierarchical thermodynamics. For example, insofar as cells live for a far shorter time than organisms, one may consider that the organisms' (organ's) medium for all practical purposes does not change during the lifetime of many types of cells. This medium fulfills the role of a thermostat for the quasi-closed subsystem (system) of the organism - cells.
It is necessary to bear in mind that each species of living being (tissue, types of cell, types of organelle, etc.) is characterized by its lifetime values of the elements of the different hierarchical structures. However, for all lower level hierarchies of living systems, which is part of a higher level hierarchy (population, organism, cell, supramolecular formation, and so on), series (1) is fulfilled.
This law can be formulated in another way: "Any living system of any temporal hierarchical level in a normal state has a thermostat - a surrounding medium that is characterized by slightly changing average values of thermodynamic parameters."
The main reason for this statement is connected with the phenomenon of metabolism and the exchange of mater of different hierarchies. Lower level hierarchical structures are often reproduced in a medium of higher level hierarchical structures during the lifetime of the latter. Thus, we have:
where ti - average lifetime of structures of lower hierarchical level, ti+1 - average lifetime of structures of higher hierarchical level.
The existence of law (1-2) allows us to use quasi-closed thermodynamic models to investigate living systems.
It should be remembered that every species of living beings (like certain tissue types) has its own characteristic lifetime of elements belonging to different hierarchic structures.
However, the law (1-2) is valid for all lower hierarchic systems involved in a higher hierarchy (e.g., supramolecular aggregate, cell, organism, population, and so forth). For example, this law is valid for bacteria whose life span is close to 20 minutes; for a moth who lives for one day; for a fly living for about one month; for a mouse living for about three years; for a dog living for about 20 years; for a human living for about 100 years, etc.
Thus, a series of the type (1) sometimes can be characterized by the geometric series tn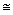 to to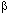 n, where tn is the mean lifetime of temporal structures of the n-th hierarchies in a particular hierarchic series of structures; to and n, where tn is the mean lifetime of temporal structures of the n-th hierarchies in a particular hierarchic series of structures; to and 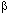 are constants for the particular series; n = 1,2,3, ... , n. are constants for the particular series; n = 1,2,3, ... , n.
The findings of macrothermodynamics (supramolecular thermodynamics) of quasi-closed systems and the published data about the variation of the chemical composition of living organisms (tissues of organisms) in ontogeny confirm the thermodynamic tendency of aging processes. According to the theory the specific value of the Gibbs function of the formation of supramolecular structures of the organism tends to a minimum:
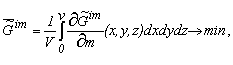
where V is the volume of the system; m - the mass of the selected micro-volumes; x, y, and z are coordinates.
The symbol "-" means that we consider the specific value of G ( 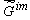 ) ; the symbol "~" stresses that the system is heterogeneous. ) ; the symbol "~" stresses that the system is heterogeneous.
That tendency explains the variation of the supramolecular and chemical composition and the morphology of tissues during aging.
The law of temporal hierarchies allows us to create quasi-closed (thermodynamic and kinetic) models of biological systems. The simple example of that type model is a chromatographic column.
The chromatographic method is based on the separation (the partition) of chemical substances. It is linked to the separation of the substance being examined into two phases - stationary (immobile) and mobile. The stationary phase in the chromatographic column is usually an adsorbent with a developed surface.
The mobile phase - a stream of gas or liquid (fluid), which filters (moves) through the layer of adsorbent. A typical chromatographic system consists of a column containing adsorbent, and a liquid substance or a solution (a mixture of different substances), flowing into the column.
Let's ask a naive question. Is the chromatographic column an open system in itself? The answer is clear; it stands to reason that this is an open system!
If the adsorbent in the column has certain catalytic activity qualities, then such a column may sustain chemical reactions between components of the solution (gas) entering the column. It is also possible that there will be chemical interaction between the components of the mobile and stationary phases.
In this way, such a column may sustain chemical reactions, which also have their place in living organisms.
Any educated biologist, chemist or physicist will be well aware that processes occurring in effective chromatographic columns are with a good approximation, in a state of thermodynamic equilibrium. In other words, such chromatographic columns are quasi-equilibrium steady state systems.
It is possible to regard an organism's tissues as systems containing a multitude of micro-chromatographic columns. Moreover, the nature of both the stationary and mobile phases of these columns differs substantially. At the same time, the effectiveness of such natural micro-columns must be extraordinarily high.
When moving in the stream of the mobile phase, the components of the original mixture, and also the substances synthesized in the column, move along the column at differing rates. These rates are in inverse proportion to the distribution constant K of the substances undergoing chromatography. Components with a high affinity to the stationary phase, whose distribution constant significance is high, move along the column more slowly than components with a low affinity to the stationary phase. The fastest mover along the column is the component with the smallest K rating.
If we investigate the action of the column using the length of the delay-time or retention time (the length of time the substances in question remain in the column) 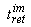 , then it is convenient to use the well-known correlation , then it is convenient to use the well-known correlation
where A - coefficient which changes evenly during the evolution of the composition in the stationary phase, for example, the biosystem (each micro-volume is characterized by its coefficient A), R- gas constant, T- absolute temperature, 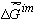 - the specific (averaged by volume or mass) Gibbs function (the Gibbs free energy) of formation of supramolecular (intermolecular) structures, appearing as a result of interaction between particles found in the mobile and stationary phases. - the specific (averaged by volume or mass) Gibbs function (the Gibbs free energy) of formation of supramolecular (intermolecular) structures, appearing as a result of interaction between particles found in the mobile and stationary phases.
The sign "-" means (as before) that the specific value is considered and the sign "~" points out the heterogeneous nature of the system.
If substances with a high affinity to the sorbent are injected into the column or are synthesized in the column continuously, then they will accumulate in the column. Such a column, whose components' concentration increases in the column according to their nature, may be regarded as partially kinetic quasi-closed. This column model, as a living system, is enlarged and its volume and mass are increased.
If the substances accumulating in the column are capable of reproducing themselves, then their concentration in the system will grow rapidly, a process which will noticeably change the chemical and supramolecular compositions of the substances, playing a role in the stationary phase.
It is also possible to use chromatographic methods to separate organelles, cells and other bio-structures. The chromatographic systems studied by chemists are analogues of living systems.
The application of classical thermodynamic methods in order to investigate dynamic systems under consideration is entirely correct independent of whether they are stationary, quasi-stationary or non- stationary! Moreover, it is obvious that the equilibrium separation of substances in the column does not depend on the degree of non-equilibrium of the chemical transformations in the column, if applicable.
As far as studying the dynamics (kinetics) of changes in a substance's nature in the biological "columns" is concerned, it is advisable to investigate the changes 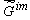 during ontogenesis, phylogenesis or evolution of supramolecular structures. It is understood that such an approach gives no information regarding the mechanism of the process. However, it does give information regarding the extent (degree of advancement) of the processes, for example, such as that of tissue aging in living organisms. during ontogenesis, phylogenesis or evolution of supramolecular structures. It is understood that such an approach gives no information regarding the mechanism of the process. However, it does give information regarding the extent (degree of advancement) of the processes, for example, such as that of tissue aging in living organisms.
However, one must not forget that rational application of the methods of equilibrium thermodynamics in order to investigate the open systems under consideration needs certain limiting conditions to be fulfilled.
One of the fundamental conditions is connected with the constancy (invariability) of the concentration of components of the solution or gas entering the column.
This condition, as is obvious from the standpoint of the thermodynamic theory of evolution and aging of living beings, needs the provision of a thermostat (in the wider sense of this term, as used in physics) for the open system. In fact, this thermostat is the environment that surrounds this very system.
This thermostat is characterized by constancy (invariability) in all thermodynamic parameters. These parameters are temperature, pressure, intensity of physical fields, concentration of chemical substances, etc. We note that in the thermostats of living systems, these parameters, although they vary in adaptive zones, retain more or less constant values for comparatively long periods of time. This is a consequence of the general law of Nature - the law of temporal hierarchies in the biological world.
Let's also direct the attention of the reader to the fact that with respect to chromatographic separation of substances, even chemical laboratory conditions can support the values of temperature, pressure and other parameters relating to the environment surrounding the column with limited precision. It is understood that this precision corresponds in part to the lesser variations with respect to different parameters of the thermostat than is the case with many real biological systems, which exist for any length of time. All the same, in all such cases, we are dealing with one or another open system model - models that are studied using the methods of equilibrium thermodynamics.
For clarity, I note that there are many open geological systems in Nature that can be investigated by the quasi-equilibrium thermodynamic methods of quasi-closed complex systems. For example, the separation of minerals (gold, quartz and so on) takes place in rivers under the action of gravitational forces. For those quasi-closed systems it is truly:
| |
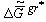 |
<0 |
|
|
were 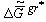 - the variation of gravitational component of Gibbs function of complex thermodynamic system. - the variation of gravitational component of Gibbs function of complex thermodynamic system.
Thus, when determining the direction of evolution of bio-systems on a chemical and supramolecular level, the "surprisingly incomprehensible" complexity of structure of chromatin, organelles, cells, and any other biological objects has no significance. The model presented in our work supports the action of the laws of thermodynamics in each local volume of supramolecular structures and, in the final analysis, in any (according to size) macro-volume biological mass.
The calculations performed are found to fully correspond with the theory and all facts known to the author.
On the basis of the thermodynamic theory of aging, notions of genes' supramolecular thermodynamics have been formulated. The possibility of a delicate interference of chemical substances in the functioning of genetic apparatus at the supramolecular level has been substantiated. The directed change of the supramolecular stability of the genetic apparatus enables it to positively influence the aging processes of organisms.
The theory makes it possible to define the principles upon which proper diets and medications can be devised to slow down aging:
"Diets incorporating "thermodynamically evolutionary juvenile" foods of vegetable and animal origins facilitate longevity and improve the quality of life. The extent of "evolutionary juvenility" of a natural foodstuff is determined by its chemical composition and supramolecular structure, which in turn depend on its phylogenetic and ontogenetic age, and also on the habitat of the organism - the source of the foodstuff."
The value of the specific Gibbs function of the formation of the supramolecular structure is an important quantitative measure of the "Gerontological value" of a natural foodstuff.
Such proper diets and medications are also useful in preventative care and in the treatment of various pathologies and among them that attending old age.
The principle of the stability of chemical substance of the supramolecular structures of tissues and more general principle of "the stability of hierarchical matter" make it possible to understand the causes of the practically unlimited evolution of the biological world from the perspective of the second law in its classical definition.
This principle can be defined as follows: "During the formation of the comparatively more stable structures of the higher hierarchical level (j+1) nature spontaneously prefers to use the comparatively less stable structures of lower hierarchical levels (j)".
Applying hierarchical thermodynamic method to the quantitative study of quasi-closed biological systems, one can develop a theory based on physical grounds that would describe their behavior on all levels, including the social and ecological ones. It can be shown that the Weber-Fechner law is a thermodynamic law. It can be understood from a point of view of the La Chatelier-Brown principle which is applied to equilibrium closed (quasi-equilibrium quasi-closed) systems.
Now I would like to say about the model of chirality formation. A model of arising of asymmetry in the bioworld is proposed. It is supposed that chemical evolution on Earth may have started from the uniform distribution of enantomorphous forms of substances. It is postulated and substantiated the role of Earth rotation in the selection of asymmetry signs of living and inorganic macro-structures (higher levels hierarchical structures) could lead to the selection of asymmetric substructures (i.e. microstructures forming macro-structures) including D- and L- forms of molecules.
It was established that the evolution is described both by thermodynamic and kinetic factors and by the trajectory factor (symmetry or chirality) which should be accounted for in rotating coordinate systems. The Coriolis forces can support spiral bio-structures to a different degree depending on the habitation area of organism.
The time of thermodynamic theory of biological evolution and aging has come now. All conclusions of the hierarchical thermodynamic theory of aging are in good agreement with centuries-old experience of mankind and with other reliable facts. It can be hoped that, the onset the 21st century, a thermodynamic theory should soon allow us, I believe, to "postpone" aging by 15-20 years on average, and benefit the preservation of youthfulness and health in people of any age.
Гладышев Георгий Павлович
Институт химической физики им. Н.Н.Семенова РАН
117977 Москва, ул. Косыгина, 4
http://www.endeav.org/evolut
E-mail: academy@endeav.org
(095) 939-71-65
(095) 285-53-95
References
- Gladyshev G.P. On the Thermodynamics of Biological Evolution // J. Theor. Biol. - 1978. - Vol. 75. - P. 425-441.
- Gladyshev G.P. Termodinamika i Makrokinetika Prirodnykh Ierarkhicheskikh Protsessov (Thermodynamics and Macrokinetics of Natural Hierarchical Processes). - M.: Nauka, 1988.
- Gladyshev G.P. Thermodynamic Theory of the Evolution of Living Beings. - M.: Luch, 1996.
- Gladyshev G.P. Thermodynamic Theory of the Evolution of Living Beings. - N.Y.: Nova Sci. Publ. Inc., 1997.
- Gladyshev G.P. Thermodynamics of Aging. (Materials for the Symposium "Thermodynamics and Information Theory in Biology" - 1998 AAAS Annual Meeting and Science Innovation Exposition AAAS's 150-th Anniversary Celebration, 12-17 February - Philadelphia, Pennsylvania, Monday, February 16, 3:00pm-6:00pm, Track: Emerging Science: Transforming the Next Generation), Report. - 1998.
- Gladyshev G.P. On the thermodynamics, entropy and evolution of biological systems: What is life from a physical chemist's viewpoint // Entropy. - 1999. - Vol. 1. - N. 2. - P. 9-20. www.mdpi.org/entropy
- Gladyshev G.P. Thermodynamic theory of biological evolution and aging. Experimental confirmations of theory. // Entropy. - 1999. - Vol. 1. - N. 4. - P. 55-68. www.mdpi.org/entropy
- Gladyshev G.P. Thermodunsmic Theory of Aging Explains Reasons of Aging and Death With Standpoint of the General Laws of Nature // Adv. Geront. - 2001. - Vol. 7. - P. 42-45.
- Gladyshev G.P. Supramolecular thermodynamics is a key to understanding phenomena of life. What is life from a physical chemist's viewpoint. - M.: Lermontov`s Fond, 2001.
- Gladyshev G.P. The Hierarchical Equilibrium Thermodynamics of Living Systems in Action // Seed Journal. - 2001.
й Г.П.Гладышев, 2001
УДК 536.7+531.3:541.1:575.8001.57; 576.1:577:577.3+577.1+577.7+577.42
Georgi P. Gladyshev
THERMODYNAMICS OF BIOLOGICAL EVOLUTION AND AGING
Supramolecular thermodynamics is a key to understanding phenomena of life. What is life from a physical chemist's viewpoint
International Academy of Creative Endeavors, Moscow, Russia, San Diego, USA;
N. Semenov Institute of Chemical Physics, Russian Academy of Sciences,
Kosygina 4, Moscow, 117977 Russia; http://www.endeav.org/evolut E-mail: academy@endeav.org
The law of temporal hierarchies of the biological world allows us to pick out of the biomass quasi-closed thermodynamic systems with a given hierarchy. It has been established, that the use of this Law of Nature as applied to supramolecular structures of organisms allows us the opportunity of using the methods of equilibrium supramolecular thermodynamics in the examination of open living systems.
It has been shown that supramolecular thermodynamics is one of the "keys", which allows us to explain the origin of life and evolution of living beings.
The second law of thermodynamics in its classic formulation (R.Clausius & J.W.Gibbs) is easy to apply in order to make calculations, carried out through methods of chemical, supramolecular and overall hierarchical thermodynamics.
Страница 260 
|



 Articles
Articles